FDA Approves First Ever Apple AirPods As Hearing Aids
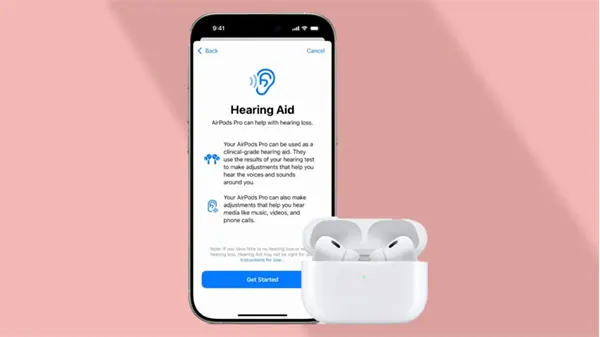
Apple AirPods Pro 2 became the first-ever over-the-counter gadget to get FDA approval as hearing aids. The announcement was made on September 12, 2024, by the Food and Drug Administration, and will take effect soon on selective Apple devices through a software update.
It will aid many people with mild hearing loss by amplifying specific sounds and minimizing external noise. The users will have to take a hearing test in the Apple Health app, and based on those results, the AirPods will automatically adjust the sound levels.
FDA in its official statement said that Apple’s hearing aid feature was approved after a rigorous clinical study with 118 subjects with moderate hearing issues. The people found similar improvements in their conditions as when using professional hearing aids.
For now, this feature is only available on Apple AirPods Pro 2, priced at $249. It has advanced features for monitoring breathing disturbances and sleep apnea notifications. It will be available in over 150 countries and cities, including the U.S., the EU, and Japan.
It is a big leap of success for the tech giant, that has always advocated its commitment to health with experiences for its users. After the official announcement, the social media buzz hasn’t stopped praising the company’s efforts, and that too at a comparatively budget price tag.
As we wait for more Apple innovations, this one will be recorded in history as an example of a successful collaboration between the tech and health industries.